Nitrogen Atom

Last updated on February 15th, 2020
Nitrogen is a chemical element with atomic number seven and atomic weight 14. Its symbol is N. With these facts about nitrogen, let us learn about its chemistry, physical properties, atomic mass, and much more.
26 Facts about Nitrogen Wavepad for chromebook.
2020-11-21 by Nick Connor Atomic Mass of Nitrogen Atomic mass of Nitrogen is 14.0067 u. The configuration notation for Nitrogen (N) provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of the Nitrogen atom. This makes it easier to understand and predict how atoms will interact to form chemical bonds.
1. It has a melting point of −209.86 °C (−345.8 °F) and a boiling point of −195.8 °C (−320.4 °F).[2]
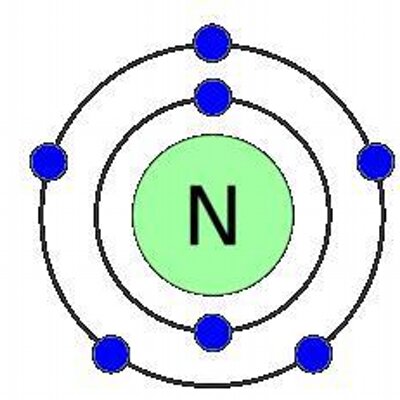
Nitrogen has 2 electrons in its first shell and 5 in its second.Check me out: http://www.chemistnate.com. Read interesting facts about the nitrogen atom, liquid nitrogen, nitrous oxide, nitric acid, nitroglycerin and much more. Nitrogen is a chemical element with the symbol N and atomic number of 7. Under normal conditions nitrogen is a colorless, odorless and tasteless gas. Nitrogen makes up around 78% of the air you breathe.
2. Nitrogen which is a non-metal was named nitrogen by a French Chemist Jean-Antoine-Claude Chaptal in 1790. The chemist realized that the gas was a part of a chemical compound then called niter, which we now refer to as potassium nitrate.[2,7]
3. It is the sixth most abundant element in the cosmos.[2]
4.By volume, nitrogen gas makes 78.09 %, and by weight, it makes 75.51% of the atmosphere. Comparatively, oxygen comprises approximately 21% of the earth’s atmosphere.[2]
| Name of the gas | % by weight in air |
|---|---|
| Oxygen | 20.99 |
| Nitrogen | 78.01 |
| Carbon dioxide | 0.03 - 0.07 |
| Argon | 0.94 |
| Hydrogen | 0.01 |
| Neon | 0.0015 |
| Helium and Krypton | 0.01 - 0.02 |
5. Nitrogen is commercially produced by the fractional distillation of air.[2]
6. Scottish chemist Daniel Rutherford isolated nitrogen for the first time in 1772. Rutherford removed oxygen and carbon dioxide from the air and showed that the remaining gas in the air would not support combustion or living organisms. Thus, the credit for the discovery of the element is generally given to him.[3]
7. The nitrogen cycle is one of the most important processes in nature for living organisms.[5]
8. Nitrogen has two stable isotopes: nitrogen-14 and nitrogen-15. Almost all (99%) of the nitrogen in the universe is nitrogen-14.[3]
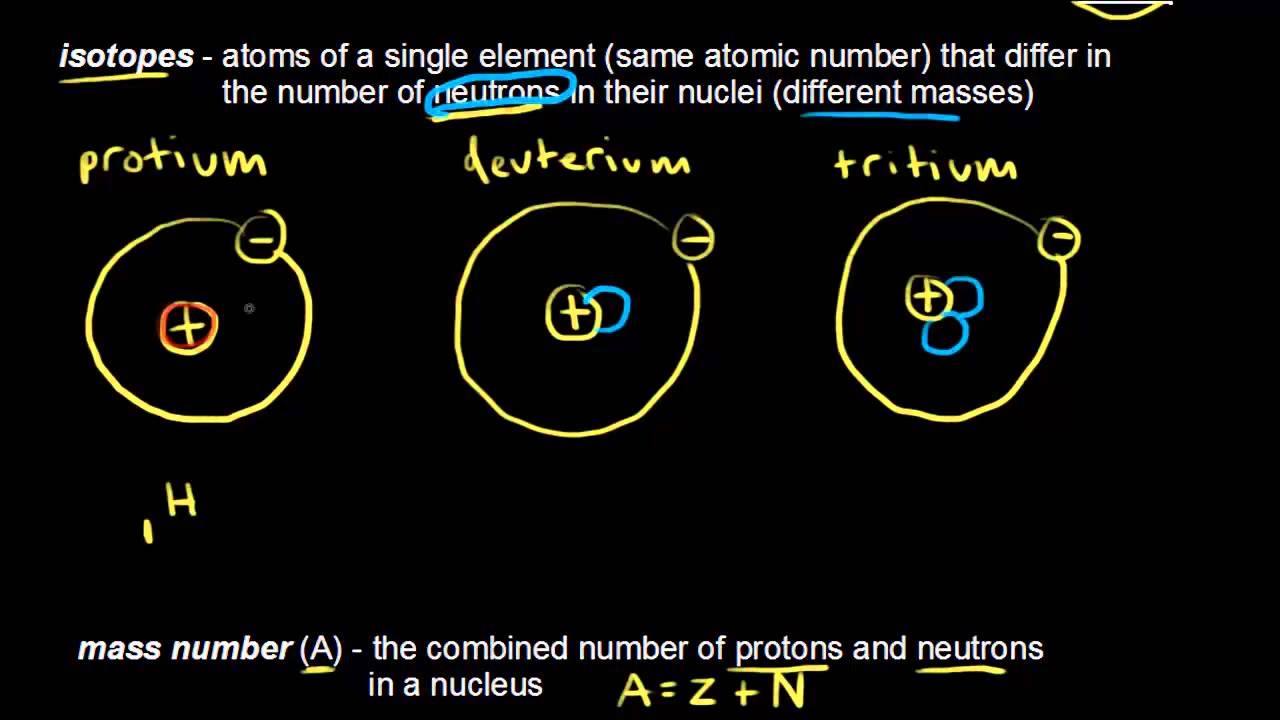
9. It is the lightest member of group 15 of the periodic table.[1]

10. Nitrogen is also present in the human body. In fact, it is the fourth most abundant element (after oxygen, carbon, and hydrogen) in the human body by mass.[3] Pandoc latex to html online.
 What is Luminar AI? Luminar AI is a new and innovative AI-powered photo-editing software that focuses on delivering outstanding results in the shortest possible time and without any prior experience with photo editing. “ Luminar AI will help make image editing easier for everyone. Luminar AI makes the kinds of changes an experienced photo editor would in a fraction of the time, from a clear, easy to use interface. You want professional results quickly The reliance on AI. With Luminar AI, you bring a whole team of experts to your editing session. Luminar AI is an all in one non-destructive photo cataloguing and cataloguing program. You can use it to browse, filter and organize your photos, and any changes you make with templates or filters can be reversed or modified at any time. Luminar AI is available both as a standalone piece of software and as a plug-in that is able to work alongside Photoshop or Lightroom. Use code NATURETTL at checkout for a £10 discount on Luminar AI.
What is Luminar AI? Luminar AI is a new and innovative AI-powered photo-editing software that focuses on delivering outstanding results in the shortest possible time and without any prior experience with photo editing. “ Luminar AI will help make image editing easier for everyone. Luminar AI makes the kinds of changes an experienced photo editor would in a fraction of the time, from a clear, easy to use interface. You want professional results quickly The reliance on AI. With Luminar AI, you bring a whole team of experts to your editing session. Luminar AI is an all in one non-destructive photo cataloguing and cataloguing program. You can use it to browse, filter and organize your photos, and any changes you make with templates or filters can be reversed or modified at any time. Luminar AI is available both as a standalone piece of software and as a plug-in that is able to work alongside Photoshop or Lightroom. Use code NATURETTL at checkout for a £10 discount on Luminar AI.
11. Nitrogen is the most common uncombined element on earth.[19]
12. Nitrogen is a poor conductor of heat and electricity.[18]
13. Under normal conditions, nitrogen is a colorless, odorless and tasteless gas.[17]
14. Ammonia (N3) a compound of nitrogen is commonly used in fertilizers.[15]
15. The atmosphere of Saturn’s moon Titan is made out of nitrogen to a large extent (almost 98%).[13,14]
16. The reaction of nitrogen with hydrogen resulting in ammonia is known as Haber Process.[5]
17. Nitrogen is used for packing bags of crisps. The gas prevents the oxidation of crisps.[16]
18. Laughing gas or happy gas, the common name of Nitrous Oxide, is used for performing some dental procedures. It is used widely as an anesthetic in both dental and medical applications. It is also used as a food preservative.[4]
19. Nitrogen is a component of all proteins.[5]
20. Interestingly, the atmosphere of Mars is only 2.6% nitrogen.[5]
21. Nitrogen is nonflammable and does not support combustion.[6]
Nitrogen Atom In Ground State
22. Did you know that Nitrogen is found is stars, animals, plants and even in our own DNA? Actually, it is a constituent element of amino acids and therefore of proteins and nucleic acids (DNA and RNA).[9]
23.Liquid nitrogen is so cold that it can even cause frostbite when it comes in contact with living tissue.[8]
24. The human body contains 3% by weight of nitrogen, making it the fourth most abundant element in the body after oxygen, carbon, and hydrogen.[10]
25. Oklahoma, a state in the U.S., has declared that it will start using nitrogen for all its executions moving forward, thus becoming the first US state to do so. Learn that capital punishment (death penalty) is still practiced in the state.[11]
Nitrogen Atom Configuration
26. Did you know that agricultural activities are responsible for about two-thirds of global nitrogen pollution? According to some estimate, 120 million tons of synthetic nitrogen is used globally in agriculture each year. The shocking truth is that more than half of this is washed off from fields into rivers leading to pollution.[12]
About Nitrogen – Quick facts and information
Nitrogen Atomic Weight
| Name | Nitrogen |
|---|---|
| Origin of the name | The name is derived from the Greek 'nitron' and 'genes' meaning nitre forming. |
| Common use | 1. Liquid nitrogen is often used as a refrigerant. 2. used for storing sperm, eggs and other cells for medical research and reproductive technology. 3. used to rapidly freeze foods, helping them to maintain moisture, colour, flavour and texture. 4. Welding 5. Hydrochloric acid 6. Reducing metallic ores 7. Filling balloons |
| Properties | 1. carbon-free 2. exceptionally clean 3. lighter than air 4. odourless 5. non-toxic 6. safe to produce, store and transport 7. easy to store in large amounts 8. easily produced from many different sources |
| Symbol | N |
| Atomic number | 7 |
| Atomic mass | 14.0067 atomic mass units (amu) |
| Discovered by | Daniel Rutherford |
| Discovery date | 1772 |
| Density | 0.07807 lb/cu. ft 1.2506 kg/m3 |
| Melting point | −209.86 °C (−345.8 °F) |
| Boiling point | −195.8 °C (−320.4 °F) |
| Family | Nonmetal |
| Number of Protons | 7 |
| Number of Neutrons | 7 |
| Number of Electrons | 7 |
| Molecular Formula | N2 |
| Molecular weight | 28.01 g/mol |
| Color | Colorless |
| Block | p-block |
| Isotopes | 16 including 2 stable ones Most common isotopes: Nitrogen-14 (Abundance: 99.63 percent) |
| Member of group | 15 |
| Allotropes | none |
| Electron configuration | [He]2s22p3 |
| Latent heat of Vaporization | 85.6 BTU/lb |
| Percentage in Atmosphere (by volume) | 78.09% |
| Phase at room temperature | gas |
| Crystal structure | hexagonal close-packed |
| Atomic Radius (the distance from the centre of the nucleus to the outermost shell of the electron.) | 155 pm (Van der Waals) |
| CAS number | 7727-37-9 |
| Period | 2 |
| Specific Gravity (Gas Phase Properties @ 32°F & @1 atm) | 0.9737 (Air=1) |
| Table data sources | 1. https://www.livescience.com/28726-nitrogen.html 2. http://periodic.lanl.gov/7.shtml 3. http://www.rsc.org/periodic-table/element/7/nitrogen 4. http://www.uigi.com/nitrogen.html 5. https://en.wikipedia.org/wiki/Atomic_radii_of_the_elements_(data_page) |
| Table last updated | August 03, 2018 |
