Number Of Electrons In Magnesium
What type of bond do magnesium and sulfur form?
1 Answer
Explanation:

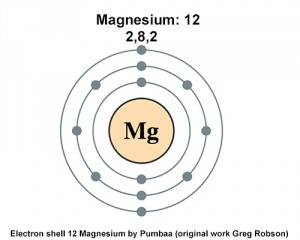
Since 1s can only hold two electrons the next 2 electrons for magnesium go in the 2s orbital. The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the remaining two electrons in the 3s. Therefore the Magnesium electron configuration will be 1s 2 2s 2 2p 6 3s 2. Magnesium: Element Name: 24.31: Average Atomic Mass: The atomic weight is basically a measurement of the total number of particles in an atom 's nucleus including both protons and neutrons. So the first step is to locate the atomic mass on the periodic table, and round the value to the nearest whole number. Therefore, the number of electrons in neutral atom of Magnesium is 12. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other (Z – 1) negative electrons in the atom.
Magnesium,
On the other hand, sulfur,
As you know, chemical reactivity is governed by an atom's 'desire' to have a stable electron configuration, that is, to have eight electrons in its outermost shell
In this case, magnesium can complete its octet by giving up those two valence electrons, becoming the magnesium cation.
Sulfur, which only needs two electrons to complete it octet, will pick up the two electrons coming from magnesium, becoming the sulfide anion,
The electrostatic force of attraction will then bring the magnesium cations and the sulfur anions together

Related questions
What are the quantum numbers of Mg?
1 Answer
Explanation:
When asked the quantum numbers of an element, the question is really asking about only the valence or outermost electron.
The four quantum numbers determine the state of the electron, and are
The electronic configuration for magnesium is
It also means that
Finally,
Putting all of these together, the final quantum numbers of a magnesium atom are
Number Of Electrons In Magnesium Ion
Related questions
